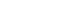Business Area
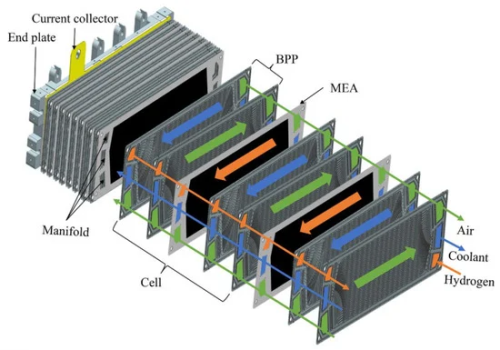
Hydrogen Fuel Cell
Fuel cells are devices that generate electricity through electrochemical redox reactions, not combustion. In short, they convert the chemical energy of fuels, such as hydrogen or methane, directly into electrical energy by combining them with oxygen.
Because the chemical energy does not need to be converted into thermal energy and mechanical energy first, fuel cells are extremely efficient. Besides minimizing energy losses, fuel cells are also less polluting than classic combustion: carbon emissions are much lower. If green hydrogen – hydrogen created using renewable energy sources - is fueling the cell, they only emit vapor and warm air.
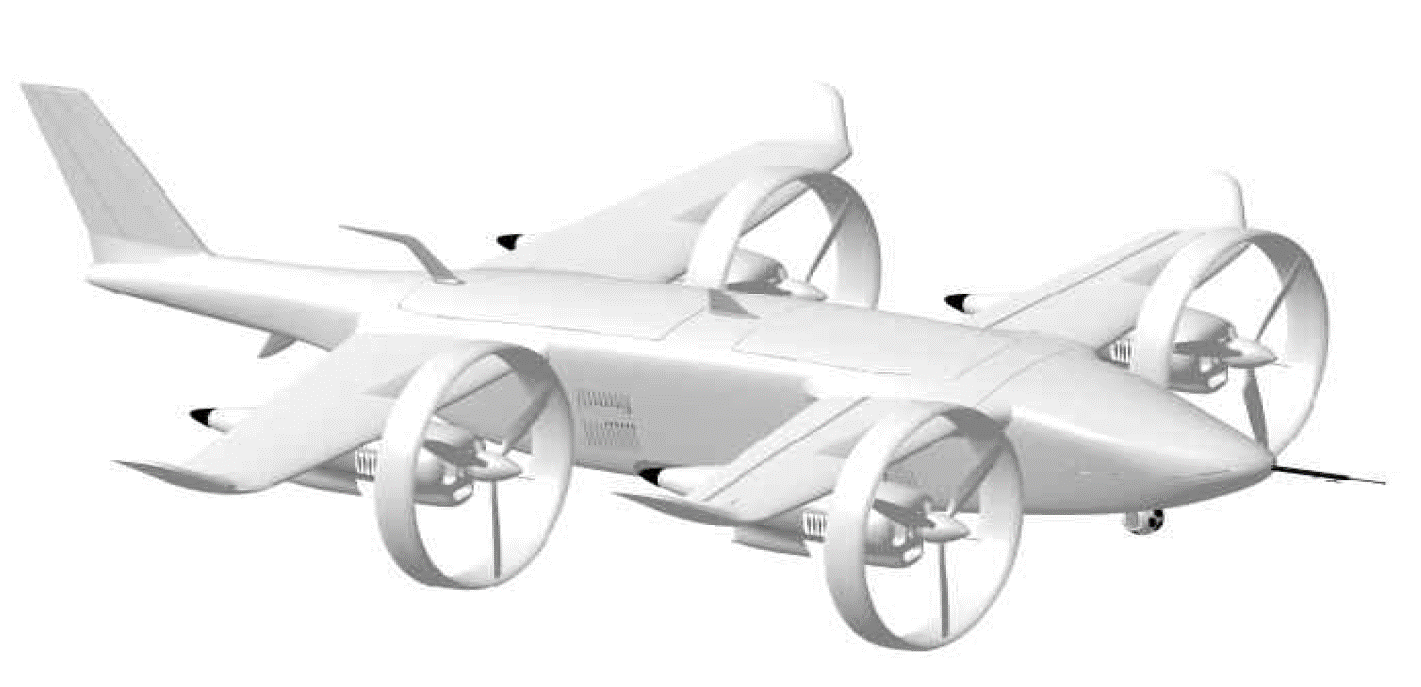
On top of that, fuel cells are allrounders. They can provide power for systems as large as utility power stations and as small as a laptops. Consequently, fuel cells are used in a wide range of sectors, including marine, aviation and railway.
Because the chemical energy does not need to be converted into thermal energy and mechanical energy first, fuel cells are extremely efficient. Besides minimizing energy losses, fuel cells are also less polluting than classic combustion: carbon emissions are much lower. If green hydrogen – hydrogen created using renewable energy sources - is fueling the cell, they only emit vapor and warm air.
On top of that, fuel cells are allrounders. They can provide power for systems as large as utility power stations and as small as a laptops. Consequently, fuel cells are used in a wide range of sectors, including marine, aviation and railway.
Electrolysis
Electrolysis is a chemical process that uses an electric current to drive a non-spontaneous chemical reaction. It involves the breaking down of a compound into its constituent elements or ions using electricity.
The overall result of electrolysis is the separation of the compound into its constituent elements or the transformation of one substance into another.
Electrolysis has various applications, including electroplating, metal extraction, water splitting, and electrolytic refining.
- The process takes place in an electrolytic cell which consists of two electrodes (an anode and a cathode) immersed in an electrolytic solution.
- The anode is the positive electrode, where oxidation occurs, while the cathode is the negative electrode, where reduction occurs.
- When an electric current is passed through the electrolytic solution, positive ions migrate toward the cathode, while negative ions migrate towards the anode.
- At the cathode, reduction reactions take place, resulting in the formation of new substances or the deposition of metals.
- At the anode, oxidation reactions occur, leading to the release of the electrons and the formation of new substances or gases.
The overall result of electrolysis is the separation of the compound into its constituent elements or the transformation of one substance into another.
Electrolysis has various applications, including electroplating, metal extraction, water splitting, and electrolytic refining.

P2X (Power to X)
Power-to-X (also P2X and P2Y) are electricity conversion, energy storage, and reconversion pathways from surplus renewable energy.
Power-to-X conversion technologies allow for the decoupling of power from the electricity sector for use in other sectors (such as transport or chemicals), possibly using power that has been provided by additional investments in generation.
The X in the terminology can refer to one of the following: power-to-methanol, power-to-ammonia, power-to-chemicals, power-to-fuel, power-to-gas (power-to-hydrogen, power-to-methane) power-to-liquid (synthetic fuel), power to food, power-to-heat. Electric vehicle charging, space heating and cooling, and water heating can be shifted in time to match generation, forms of demand response that can be called power-to-mobility and power-to-heat.
Collectively power-to-X schemes which use surplus power fall under the heading of flexibility measures and are particularly useful in energy systems with high shares of renewable generation and/or with strong decarbonization targets.
Power-to-X conversion technologies allow for the decoupling of power from the electricity sector for use in other sectors (such as transport or chemicals), possibly using power that has been provided by additional investments in generation.
The X in the terminology can refer to one of the following: power-to-methanol, power-to-ammonia, power-to-chemicals, power-to-fuel, power-to-gas (power-to-hydrogen, power-to-methane) power-to-liquid (synthetic fuel), power to food, power-to-heat. Electric vehicle charging, space heating and cooling, and water heating can be shifted in time to match generation, forms of demand response that can be called power-to-mobility and power-to-heat.
Collectively power-to-X schemes which use surplus power fall under the heading of flexibility measures and are particularly useful in energy systems with high shares of renewable generation and/or with strong decarbonization targets.

Applications
Until now, no one has been able to develop mobility applications using methanol fuel cells. The main reason is that methanol fuel, being a liquid, causes disturbances in power generation due to shaking. This problem must be solved using big data accumulated over a long period. We solved this problem 15 years ago. We have already developed many mobility applications using fuel cells. These applications include motorcycles, utility vehicles, forklifts, and even UAVs. Additionally, we have succeeded in attempting applications that no one else has tried, such as UPS, portable power generators, and megawatt power stations.
Methanol fuel cells offer a versatile and sustainable energy solution for various applications, ranging from portable electronics to transportation and remote power systems. Their high energy density, ease of storage, and potential for renewable production make them an attractive alternative to traditional power sources, despite the existing challenges. Continued research and development, along with infrastructure investment, will be crucial in realizing their full potential across these diverse applications.
Methanol fuel cells offer a versatile and sustainable energy solution for various applications, ranging from portable electronics to transportation and remote power systems. Their high energy density, ease of storage, and potential for renewable production make them an attractive alternative to traditional power sources, despite the existing challenges. Continued research and development, along with infrastructure investment, will be crucial in realizing their full potential across these diverse applications.